|
Aromaticity
|   |
The DRAGON algorithm identifies aromaticity regardless the aromatic bonds defined in the molecule input file. In fact, the user can enter a Kekulè-like structure with alternating single and double bonds or a structure as aromatic directly. DRAGON can always recognize aromatic rings only on the basis of atom valences.
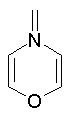
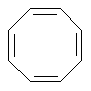
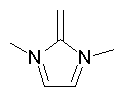
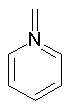
A ring is identified as aromatic if it is planar (all atoms are sp2 hybridized), doesn't form exocyclic double bonds and satisfies the Huckel's 4N+2 rule. Moreover, the only allowed heteroatoms are N, O, S, and P.
Rules for counting shared pi electrons:
For charged structures the total number of shared pi electrons is reduced by the positive in-ring charge and increased by the negative in-ring charge.
If the molecule structure is entered by SMILES notation, the DRAGON algorithm identifies pyrrolyl N (sharing 2 pi electrons) by the atomic symbol n (or N) and pyridyl N (sharing 1 pi electron) by the symbol n+ (or N+). Therefore, SMILES users are strongly suggested to always specify pyridyl type nitrogen in order to allow DRAGON to correctly represent the molecule structure.
If two bonds formed by a sp3 N are originally marked as aromatic, this N is by default identified as pyridyl type. However, if by this assumption the structure does not result aromatic, then the pyrrolyl N hypothesis will be tested. In any case, if the structure is not aromatic a nitrogen with valence greater than 3 and lower than 5 is considered as N+.
If a structure exists in tautomeric forms and the user has entered the nonaromatic one, DRAGON algorithm cannot recognize it as aromatic.
Note that the DRAGON algorithm for aromaticity detection doesn't pretend to account for real molecule aromaticity. It is based on some rigorous rules which have been implemented to recognize most of the common aromatic structures.
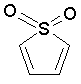
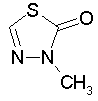
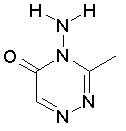
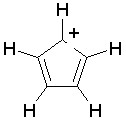
Some examples of aromatic and nonaromatic structures as recognized by DRAGON are reported below.
Examples of aromatic structures:
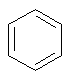
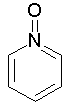
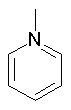
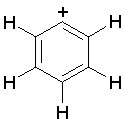
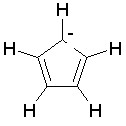
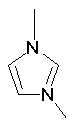
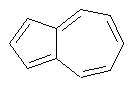
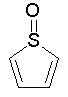
Examples of nonaromatic structures: